INTERACTION BETWEEN CLAYS AND POLYCARBOXYLATE SUPERPLASTICIZERS

The robustness of PolyCarboxylate Ether (PCE) superplasticizer performance is challenged by the variable amount of clay minerals in aggregates. It will be shown here how a swelling clay like Montmorillonite interacts with polymers, interfering with their ability to disperse cement particles thus influencing the rheological behavior of concrete. Here, the mechanisms governing this interaction will be unveiled using different analytical methods.
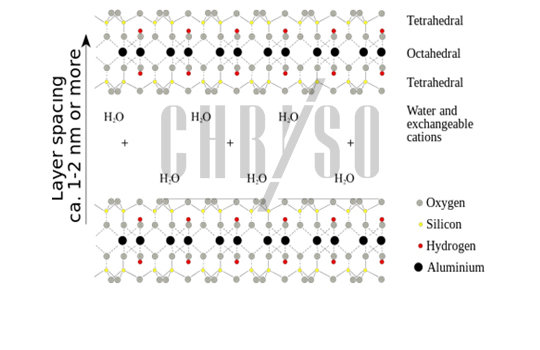
Montmorillonite is a phyllosilicate, a “leaf-like” material. It is composed of aluminosilicate stacked-together sheets, forming an “interlayer space”, filled with water and cations (positive ions)—see Figure 1.
Phyllosilicates can exchange cations when mixed with a solution containing a cation of a different nature and they may also intercalate polymers, leading to a deformation of the interlayer space to accommodate such large molecules.
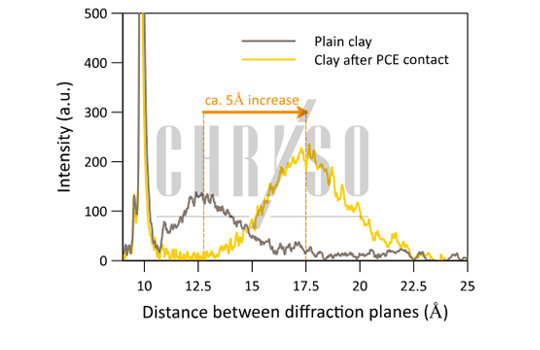
This is measurable in an X-ray diffraction experiment performed on a Montmorillonite sample dried after contact with a PCE solution. The distance between the mineral sheets increases from around 12.5 Angströms for plain clay to 17.5 Angströms, a 5-Angström stretch resulting from polymer intercalation, as shown by Figure 2.
The interlayer space can intercalate huge amounts of PCE, inducing a very strong consumption of superplasticizer and cancelling out its dispersing effect. The dosage rates must then be increased to bring some excess PCE in the mix and recover some workability.
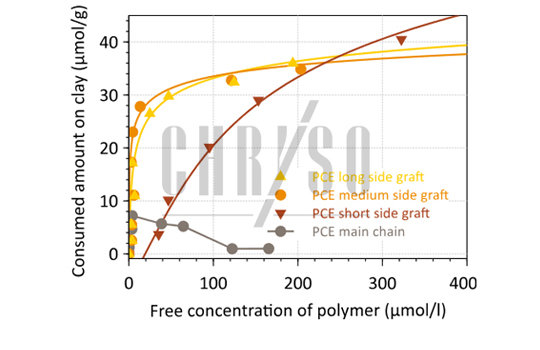
CHRYSO studies 1,2 performed by Total Organic Carbon analysis on separate main chains and side grafts of PCE could show that the whole molecule is not fully responsible for this unwanted interaction: the side-grafts of the comb molecule are particularly favored by the clay—Figure 3.
The same plot shows that all side grafts are not equal in the process, since short polymers feature a lower consumption by Montmorillonite, at least for low concentrations.
This consumption is due to a so-far overlooked mechanism involving the side-graft complexation of the interlayer sodium cations present in Montmorillonite.
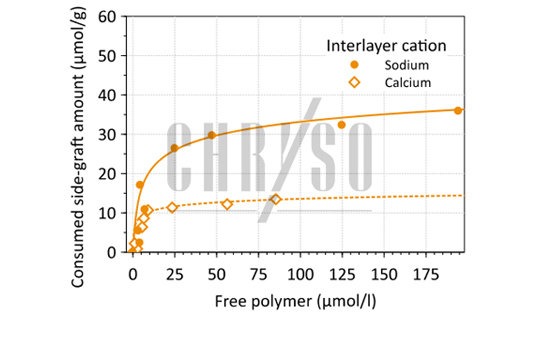
Indeed, when these sodium cations are exchanged with calcium, the interaction between Montmorillonite and side-grafts diminishes noticeably (see Figure 4), leading to a higher PCE concentration available for cement dispersion.
This also shows that not all clays are detrimental to PCE efficiency, since it depends on the cation that is naturally present in the mineral.
This fundamental knowledge allowed the R&D teams at CHRYSO to create more accurate clay-assessment protocols and better tailor new admixture solutions for clay-laden aggregates, leading to the CHRYSO®Quad CLEAR technology.
Pascal BOUSTINGORRY – PhD in construction materials
References
[1] Ait-Akbour, R., Boustingorry, P., Leroux, F., Leising, F. & Taviot-Guého, C. Adsorption of PolyCarboxylate Poly(ethylene glycol) (PCP) esters on Montmorillonite (Mmt): Effect of exchangeable cations (Na + , Mg 2+ and Ca 2+ ) and PCP molecular structure. J. Colloid Interface Sci. 437, 227–234 (2015).
[2] Ait-Akbour, R., Taviot-Guého, C., Leroux, F., Boustingorry, P. & Leising, F. Interaction of montmorillonite with poly(ethylene Glycol) and poly(methacrylic acid) polymers. Consequences on the influence of clays on superplasticizer efficiency. in American Concrete Institute, ACI Special Publication 2015–Janua, (2015).





